43 fda approved health claims on food labels
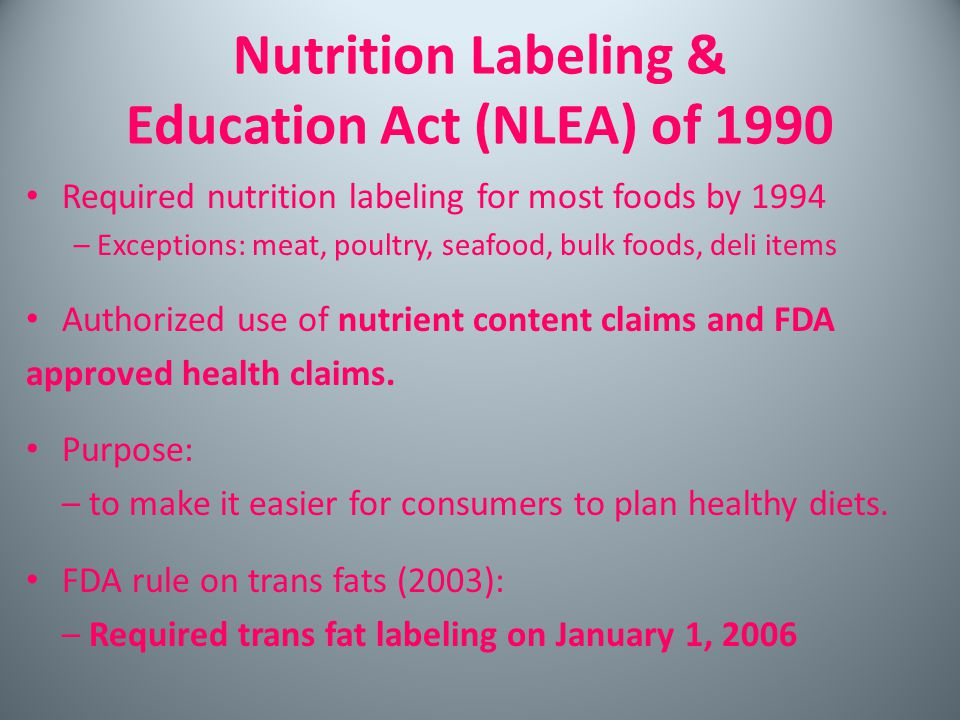
Understanding Health Claims on Food Labels - Food Smart Colorado Health Claim Definitions In addition to the nutrition facts and ingredient information found on packaged foods, some foods may also be labeled with health-related claims. There are three main categories of claims defined by and regulated by the Food and Drug Administration (FDA): Factual Food Labels: Health Claims - University of Texas at Austin According to the United States Food and Drug Administration (FDA) there are only three categories of claims that are approved to be printed on food packaging: health claims, nutrient claims, and function claims. Generally, these labels are found on the front side of the food package in emphasized lettering. Health Claims
FDA approves cardiovascular health claim on certain oil labels ... The FDA-approved health claim is as follows: ... Further, this oil label update comes as a part of the FDA's new strategy to modernize and prioritize health claims on food labels. They also aim to ...
Fda approved health claims on food labels
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Mar 29, 2022 · (4) For dietary supplements, claims regarding calories may not be made on products that meet the criteria in § 101.60(b)(1) or (b)(2) for "calorie free" or "low calorie" claims except when an equivalent amount of a similar dietary supplement (e.g., another protein supplement) that the labeled food resembles and for which it substitutes ... FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ... Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims.
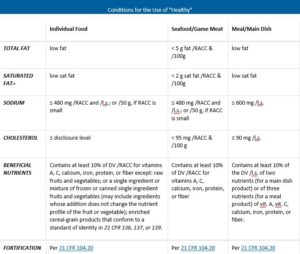
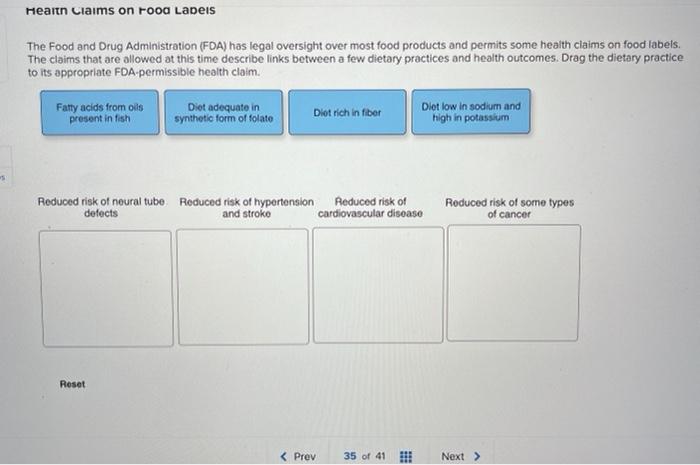
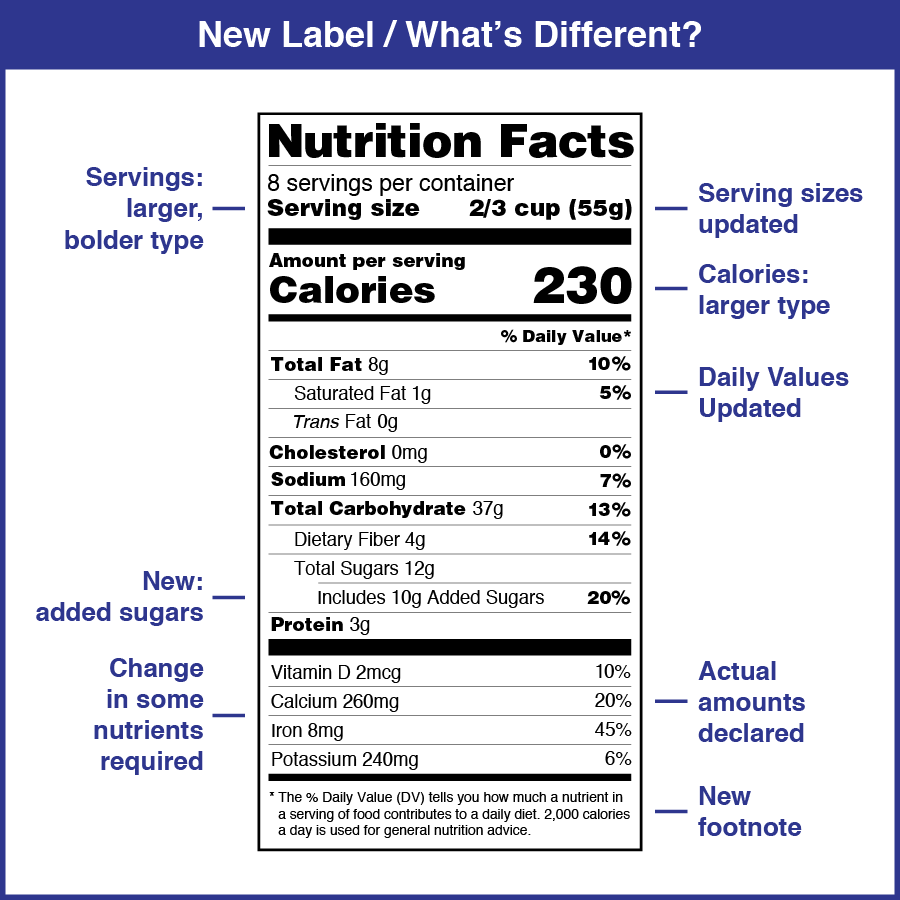
Fda approved health claims on food labels. Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food... Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... Understanding Food Labels | The Nutrition Source | Harvard T.H. Chan ... The Nutrition Labeling and Education Act of 1990 regulates these health claims, which must undergo review by the FDA through a petition process. The FDA has approved 12 health claims on food labels such as the relationship between calcium and osteoporosis; sodium and hypertension; fiber-containing grains, fruits and vegetables and cancer; and ... Health Claims on Food Labels - Consumer Reports Specifically, grass-fed meat and dairy has a more healthful ratio of omega-6 polyunsaturated fatty acids to omega-3s. Too much omega-6 fat in your diet can cause inflammation, which may be a ...
How FDA Failures Contributed to the Opioid Crisis The Food, Drug, and Cosmetic Act requires drug manufacturers to demonstrate that their products are both safe and effective before they are marketed. 13 The benefits of a drug must outweigh potential risks for specific indications listed on an FDA-approved label. 13 Although prescribing medication for unapproved uses is common and sometimes ... Qualified Health Claims: Letters of Enforcement Discretion | FDA Letter Updating the Qualifying Level of Oleic Acid for the Oleic Acid From Edible Oils and Coronary Heart Disease (Corbion Biotech Petition) Qualified Health Claim July 16, 2020. Folic Acid ... Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim" A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."
Nutrient Content Claims | FDA - U.S. Food and Drug Administration Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ... Understanding Food Labels | The Nutrition Source | Harvard … The FDA has approved 12 health claims on food labels such as the relationship between calcium and osteoporosis; sodium and hypertension; fiber-containing grains, fruits and vegetables and cancer; and folic acid and neural tube defects. However, just because a food contains a specific nutrient that is associated with a decreased risk of disease ... Is It Really 'FDA Approved'? - U.S. Food and Drug Administration May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ... Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...
Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Factual Food Labels: Health Claims - 100% Online According to the United States Food and Drug Administration (FDA) there are only three categories of claims that are approved to be printed on food packaging: health claims, nutrient claims, and function claims. Generally, these labels are found on the front side of the food package in emphasized lettering. Health Claims. In 1990, the Nutrition ...
Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Mar 29, 2022 · (a) Applicability. Except as provided in this section, this part applies to all clinical investigations of products that are subject to section 505 of the Federal Food, Drug, and Cosmetic Act or to the licensing provisions of the Public Health Service Act (58 Stat. 632, as amended (42 U.S.C. 201 et seq.)). (b) Exemptions. (1) The clinical investigation of a drug …
Food and Drug Administration - Wikipedia The United States Food and Drug Administration (FDA or USFDA) is a federal agency of the Department of Health and Human Services.The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, …
Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...
Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".
Food labeling: health claims; plant sterol/stanol esters and coronary ... The Food and Drug Administration (FDA) is authorizing the use, on food labels and in food labeling, of health claims on the association between plant sterol/stanol esters and reduced risk of coronary heart disease (CHD). FDA is taking this action in response to a petition filed by Lipton (plant ster …
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...
Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims.
FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Mar 29, 2022 · (4) For dietary supplements, claims regarding calories may not be made on products that meet the criteria in § 101.60(b)(1) or (b)(2) for "calorie free" or "low calorie" claims except when an equivalent amount of a similar dietary supplement (e.g., another protein supplement) that the labeled food resembles and for which it substitutes ...









:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652082/healthy-choice.0.png)



















Post a Comment for "43 fda approved health claims on food labels"